WGs updates
- News
- July 10, 2021
- Working Group 1
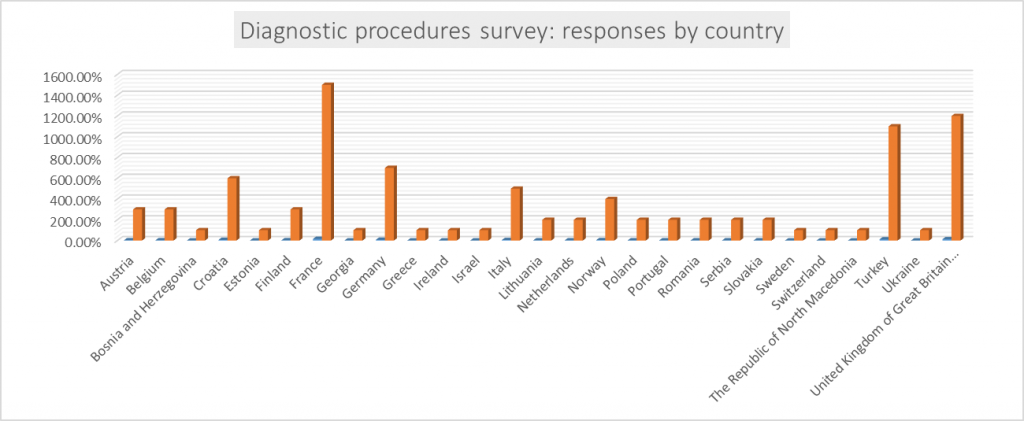
Work is ongoing in WG1 continues on dissemination of two surveys. The Diagnostic procedures survey, aims to review and evaluate the methodology and interpretive criteria used by veterinary diagnostic laboratories across Europe for pathogen identification and antimicrobial susceptibility testing. So far, the most responses for this survey came from France, UK and Turkey. We thank everyone who helped with dissemination this survey so far and please continue to so throughout your networks so we can see more and more responses and countries added to the list below.
The survey can be accessed here until the end of July.
The treatment guidelines survey aims to map and compare the availability, structure, and evidence-base of veterinary antimicrobial treatment guidelines. We had many enthusiastic responses for translation of this survey in several languages which are now available on the ENOVAT WG1 page (please see the links below); we are very grateful to all ENOVAT members who have contributed to this effort. We need to reach as many practising veterinary practitioners as possible who are working in first opinion, referral settings in the private sector and academia throughout Europe. As such, the survey will be extended beyond the 16th of July until further notice. Please help us to spread the word and help to disseminate the survey links. All information is available here.
- Working Group 2
The first objective of WG2 is to establish a database with information on veterinary pathogens stored across Europe. In collaboration with WG1 and WG3, we defined animal pathogens of the greatest interest for ENOVAT. With the help of this strain database, we will get information about the availability of already cryopreserved pathogens that are
- traditionally difficult to speciate (e.g. non-fermentative Gram-negative bacteria) or completely unidentified pathogens with MALDI TOF MS technologies, and
- pathogens, for which animal-specific Clinical Breakpoints (CBPs) and even Epidemiological Cut Off values (ECOFFs) for interpretation of antimicrobial resistance testing (AST) are lacking.
The isolates will be kept in their original place, but information about their origin, year of isolation, method of identification, etc. will be added to the database. This information will help us identify animal pathogens available in sufficient numbers across Europe to further optimize identification with MALDI TOF MS technologies or establish ECOFFs values. Laboratories that provide data on interesting strains will be contacted for further actions.
If your laboratory is willing to make a valuable contribution to the “European strain database”, please use this template format! Fulfilled excel sheets could be sent to the WG2 leaders: Gudrun Overesch (gudrun.overesch@vetsuisse.unibe.ch) and/or Annet Heuvelink (a.heuvelink@gddiergezondheid.nl).
If you are aware of other relevant laboratories in your country, for which participation could also be of interest, please forward this information and the link(s) to any laboratory that could give support.
- Working Group 3
The deliverable n. 2 of ENOVAT: report with a priority list of clinical breakpoints (CBPs), and strategy for obtaining required data set by February 2021 has been postponed and will be done before the mid of December 2021.
A survey was created on “what CBPs are missing?” and was circulated within the ENOVAT mailing list.
A total of 137 replies were obtained, most respondents were microbiologists, followed by pharmacologists and clinicians in small or large animal practice. CBPs were principally suggested for dogs and cats, followed by cattle.
In a dog, horse, swine, and cattle sulphonamide + trimethoprim was the most suggested antimicrobial for CBP definition, whereas in cat clindamycin and in poultry amoxicillin. CBP definition for drugs combination is problematic both for microbiological and pharmacological issues. Anyway, it will be explored if CBPs for sulfa/trimethoprim would be feasible and interesting.
Two almost finalised CBP based on VetCAST work will be in the list of the final 3:
- Doxycycline in pigs against Actinobacillus pleuropneumoniae and Pasteurella multocida
- Amoxicillin-clavulanic acid in dogs against Staphylococcus pseudintermedius, Staphylococcus aureus, and Escherichia coli (for soft tissue and urinary tract infections).
A training school organised by the WG on “Basic pharmacokinetics and pharmacodynamics – focus on antibiotics” will be held in Padua on 24-25 of August 2021 as a hybrid meeting (physical + online), satellite to the ENOVAT meeting.
- Working group 4
In February 21, the ENOVAT guidelines group (WG4) held a 3 day on-line training school in systematic reviews and the GRADE approach. The training school was hosted by WG4 chair Lisbeth Rem Jessen at the University of Copenhagen and was led by the ENOVAT methodology taskforce chairs Luis Carmo, Marnie Brennan and Helena Ferreira. The lectures and exercises were brilliantly taught by our own methodology chairs and a team of human medicine infectious disease physicians and clinical epidemiologists from ESCMID, counting Luigia Scudeller, Benedikt Huttner, Leonard Leibovici and Mical Paul, who had generously agreed to share their extensive guidelines experience and knowledge from human medicine with us. The training school was attended by 27 ENOVAT members involved in one or more of current ENOVAT drafting groups.
ENOVAT currently has 6 Antimicrobial use guidelines projects ongoing within the following topics; canine acute diarrhea, surgical prophylaxis in companion animals, post weaning diarrhea in pigs, bovine mastitis, bovine respiratory disease and poultry colibacillosis. Most projects are in collaboration with the ESCMID Study Group for Veterinary Medicine (ESGVM) and ENOVAT has recently joined the ISCAID guidelines project on canine pyoderma.
For further information of the ENOVAT guidelines project please contact WG4 chair Lisbeth Rem Jessen